Particle counters – continuous confidence for your cleanroom
It’s essential to monitor the cleanliness of environments used for the manufacture of critical products, such as cleanrooms used for the production of sterile pharmaceuticals. This monitoring process is vital within a regulatory compliance facility to ensure that the products used by the end consumer are effective, safe and free from contamination.
The new EU Good Manufacturing Practice (GMP) Annex 1 provides information encompassing various guidelines, from design and control of facilities, equipment and systems, to procedures for the manufacture of sterile products, and quality risk management. These are all aimed at ensuring that the quality of the end product is as high as possible.
Particle counters enable continuous monitoring of the production environment. This gives confidence in the integrity of a cleanroom throughout the process. Cherwell offers the BioAerosol Monitoring (BAMS) unit, for example. BAMS can detect both viable particles such as bacteria or fungus, and non-viable particles such as dust, in real-time. This real-time environmental monitoring allows any cleanroom incursions that may occur to be tackled quickly, reducing potential loss of time and costs.
Various particle counters are available, so it’s important to be fully informed when making a purchasing decision. Armed with the necessary information, you’ll be able to obtain the system best-suited to your unique facilities and production processes.
Key considerations when choosing a particle counter
1. Specifications and regulatory requirements
Particle counters can be portable or fixed units. For a portable unit, determine whether the particle counter can be used and transported easily. Then there’s the battery life: how often do those batteries have to be replaced, and at what cost? If the unit’s fixed, will it be necessary to adapt the room in which it will be installed? Also think about the ease of keeping the particle counter itself clean. Will it withstand vapourised hydrogen peroxide (VHP), isopropyl alcohol or disinfectants, for example?
It’s important to consider the different cleanroom grades (section 4.4 in the GMP Annex 1 document) and their requirements (section 9.14). Refer to information in tables 5 and 6 in Annex 1 (see below).
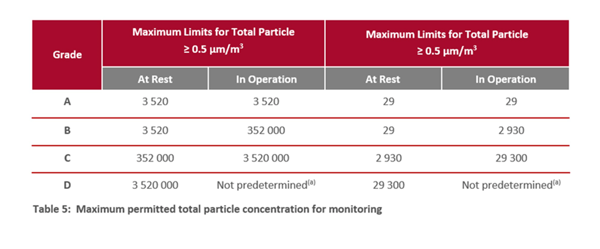
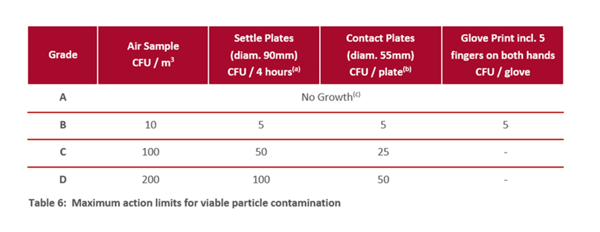
2. Measurements and particle size
Particle counters such as BAMS measure both non-viable and viable particles. A low number of viable and non-viable particles suggests a clean and well-maintained environment. To achieve a low particle count, focus on meeting the requirements of a Grade A environment, which must see no growth (Table 6, Annex 1, above). Check the particle sizes the machine can monitor: does it distinguish between microorganisms (≈1 µm) and other non-living particles such as dust?
3. Data, alerts and software
Even if the particle counter is effective, what about the data it provides? Is the information easy to understand and interpret? How is that data presented? Can it be exported, and if so, in what formats? BAMS, for example, can output data in both PDF and Excel files, which is useful to support data monitoring to understand trends and potential threats.
How does the particle counter indicate any irregularities it might detect? Does an alarm sound, or does it issue some other kind of alert? This may seem like a relatively minor issue, but the effectiveness of the alert can impact the speed with which any issues can be acted upon.
Does the particle counter require software, or licencing for software? Are there any costs associated with licensing renewals or software updates? How easy are software updates to implement? This also brings into question the unit’s connectivity, and data security issues - GMP Annex 1 and 21 CFR part 11 (Sandle, 2019).
4. Support and maintenance
Not all suppliers of particle counters are equal when it comes to aftersales support, so this is another important point. Continuous support will be useful, especially at the initial installation stage. This would enable operators to fully understand the instrument they’ll be using, and any data that may be exported for analysis. Are further training sessions available in future? Does the company offer troubleshooting support?
It’s also important to establish whether any routine maintenance is required – if so, what does this involve? Will the unit need calibration to ensure it functions correctly, reliably and accurately? Check how is this maintenance undertaken: downtime could be inconvenient and costly if the unit needs to be calibrated off-site.
Making the right choice
Particle counters represent a significant investment, so it’s important to choose the machine that’s most appropriate for your company’s cleanroom and requirements. The information above isn’t intended to be exhaustive, but should give some indication of the factors you need to take into account.
We've created a short guide that summarises things to consider when you're looking to buy a particle counter.
Buying the right particle counter from a company offering high quality support can easily be recouped in time saved, losses minimised and overall production efficiency – all of which will serve to enhance your company’s reputation. Take a look at the BAMS page for more information on Cherwell’s continuous, real-time particle counter, and if you’d like more details of our extensive range of products and services, contact us.
References
The Rules Governing Medicinal Products in the European Union. Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Annex 1: Manufacture of Sterile Medicinal Products, at: https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
https://doi.org/10.37521/ejpps.26301
Sandle, T (2019) Applying Data Integrity Principles to the Cleanroom. https://www.americanpharmaceuticalreview.com/Featured-Articles/359476-Applying-Data-Integrity-Principles-to-the-Cleanroom/
Sandle, T. (2022) EU GMP Annex 1: Manufacture of Sterile Medicinal Products. https://www.rssl.com/media/4pka2zry/rssl-white-paper-eu-annex-1-manufacture-of-sterile-medicinal-products-online-version.pdf








