Issues affecting the storage of growth media
Back in December 2017, we published a blog, taken from our guide “The pharmaceutical industry’s guide to prepared culture media” specifically about the factors to consider when storing growth media. One of the most frequent questions I am asked by customers looking for technical help are factors that affect growth media, usually accompanied by a scenario where they are worried the performance of the media is affected by a specific or number of factors. I am going to take this opportunity to address the most frequently asked questions in this area.
Ambient storage for prepared media
The shelf life of Redipor® prepared media is validated in ambient conditions. We describe ambient as 10-25°C. Hopefully the benefit is obvious, media that can be stored at room temperature does not require expensive refrigeration options and will hopefully save you plenty of storage space. You can have confidence on the performance of the media until the final day of shelf life, if stored correctly.
Our focus on ambient storage means we are slightly restricted on what media we can make. We currently do not make certain types of microbiological media, such as chromogenic agars; media containing unstable antibiotics and some blood-based agars that require refrigeration to prevent deterioration. Please contact us to check what we can make.
Out of specification storage
One of the most frequently asked questions, involves temperature excursions. We often get asked what is the effect on growth media if stored outside our stated temperature range of 10-25°C. You will be pleased to know that the answer is pretty much no effect. If you use growth media made by another manufacturer, storage is frequently stated as refrigerated (2-8°C). Sometimes in the winter during transit the temperature will fall below our minimum stated temperature of 10°C . We have successfully tested our Redipor® media by storing at refrigerated temperatures for 5 days, followed by ambient storage for the remainder of the shelf life, to demonstrate performance is not affected. The criteria are that the pH and growth promotion are identical to that of media stored without temperature excursions.
Conversely, in the summer, temperatures may exceed 25°C. We have performed similar studies for media stored at 30-35°C for 5 days followed by shelf life studies, once again performance is unaffected. I am sure that at these higher temperatures customers will be concerned about desiccation, to allay your fears the packaging will keep the moisture in. Remember also your media will most likely be incubated at anything from 30°C to 37°C or even higher. You would hope that performance is not going to be affected by heat. Just to say I hope media will never encounter extreme heat, remember agar melts at 85°C!
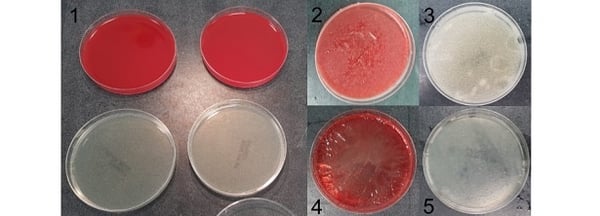
It is very unlikely that growth media is going to be exposed to temperatures below 2°C or much above 40°C. It is worth mentioning that the physical properties of agar plates are adversely affected if stored at below freezing for any length of time. You’ll know if an agar plate has been frozen, it is completely unusable (see pictures above). Picture 1 shows the TSA and Blood agar plates pre-freezing. Pictures 2 and 3 are the plates immediately after removal from the freezer. Pictures 4 and 5 are after the plates have thawed out. The damage caused by freezing and thawing is best shown on the blood agar, as you can see it is in quite a state!
Condensation in agar plate packaging
The prepared media guide mentions condensation as an issue if media is incorrectly stored. The main cause of excess condensation in packaging are rapid falls and rises in temperature. Fortunately, the effect is usually reversible. By storing at a stable ambient temperature, the condensation will be reabsorbed back into the growth media within a couple of days.
I shall save the embarrassment of a couple of customers by not mentioning names. We do have occasional complaints about frequent, excess condensation in a small number of plates. The customers said the media was stored at ambient temperature in a store room which had a reasonably stable temperature. After a bit of questioning we have discovered that some plates were stored near a radiator – removing the plates from that heat source will lead to a rapid drop in temperature, hence the condensation. Also, be warned if your store room has a window, especially if the sun shines through it. This can also be a heat source leading to increased condensation.
Transportation considerations
Existing customers know that we do not use temperature-controlled storage as standard to ship our media. The main reason is to keep costs down, temperature-controlled storage is extremely expensive and we are confident that Redipor media will still perform following temperature excursions. If it is an expectation that temperature during transport is monitored, do ask your supplier of prepared media about placing data loggers in the shipment. More specific advice concerning transport validation of medicinal products can be found in EU GMP Annex 15. Prepared media is not a medicinal product but is a critical component used as part of the testing of medicinal products and the environments they are manufactured in.
The work we have performed here at Cherwell Laboratories is not a full-scale transport validation, it is evidence of the robustness of microbiological growth media when stored at out of specification temperatures. I have found that many customers are very happy with that evidence when their auditors have questioned them about the effect of temperature changes during transport. To ensure that growth media is subject to as little temperature shock as possible, in the UK Cherwell Laboratories only ships media for next day delivery, for the Republic of Ireland shipping time is 2 days, mainland Europe is 3-4 days.
If you would like to know more about storing your prepared media, please download our eBook The Pharmaceutical and Cleanroom Industry’s Pocket Guide to Prepared Culture Media, which contains Best Practice Guidelines for Handling and Storing Prepared Culture Media. Alternatively, feel free to contact us with any queries, or to arrange a meeting to discuss your needs. We are on standby for your call.








