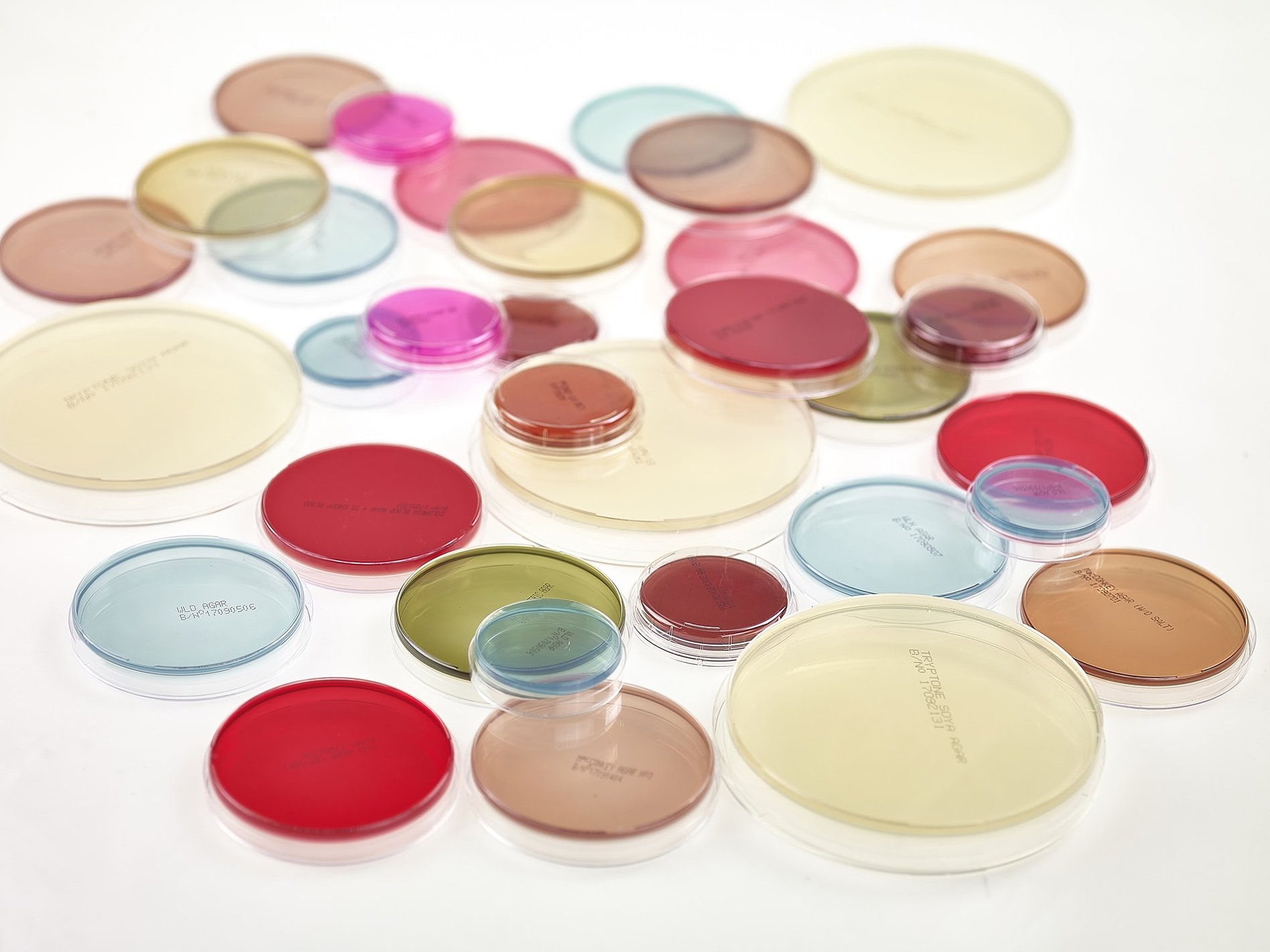
RANGE OF PREPARED CULTURE MEDIA PLATES FOR ENVIRONMENTAL MONITORING, NON-CRITICAL HYGIENE, GENERAL PURPOSE AND SELECTIVE LABORATORY MEDIA
Redipor® range of prepared media products
We provide an extensive range of Redipor® pre-poured plates including Petri dishes in 50mm, 90mm and 140mm sizes and contact plates.
Gamma irradiated products are also available.
All prepared media plates are manufactured within our cleanroom facility with quality checks at each stage of the process. Each batch is quarantined after filling, allowed to naturally condition, 100% inspected prior to final packing and can be stored at room temperature.
A sample from each batch is removed for final quality checks with a detailed certificate of analysis provided.
Browse our range of poured culture media products below and contact us to discuss any bespoke formulation requirements.
Redipor Plate Specifications Data Sheet
Safety Data Sheet - Redipor Plates

A TITLE THAT WILL DESCRIBE YOUR CONTENT OFFER
WSB - Content Section Ten
The game's not big enough unless it scares you a little. Now we know what they mean by 'advanced' tactical training. A surprise party? Mr. Worf, I hate surprise parties. I would *never* do that to you. Your head is not an artifact!
CHERWELL POURED CULTURE MEDIA PRODUCTS
Settle plates, contact plates and more: contact us to place your order
Petri dishes – 50mm
Clear polystyrene 50mm Petri dishes for use with membrane filters for applications such as water testing.
Petri dishes – 90mm
Petri dishes – 140mm
Contact plates
Dipslides
What our customers say
Tracey Roberts, Quality Assurance Technician
Wrexham Maelor Hospital
"Cherwell have always provided a great service, with innovative ideas and products. Great customer service too."
Gerald Prout
Kennet Bioservices Limited
"All my contacts with Cherwell have been resolved satisfactorily with 24-48 hours."
Microbiology Technician
Industrial Pharmaceutical Company
"The service from Cherwell is excellent, they will always try to provide help where possible."
NEED SOMETHING ELSE?
Browse our products and services
FREQUENTLY
Asked Questions
For Redipor media the Use By date is the last day on which the medium should be inoculated or exposed. Any incubation period that is normal for that product may begin on that day. For example a 14 day sterility test in TSB or a 5 day incubation for a TSA settle plate.
Dip slides can be less sensitive than contact plates or swabs and they often have less stringent QC release criteria. However there are circumstances where they have real advantages.
They are convenient and easy to use by the relatively less skilled.
They are ideal for testing water tanks and hygiene monitoring in food preparation and similarly clean but not aseptic environments. Most importantly they are ideal for field use because they are self contained and the slide can be incubated and assessed without opening the device.
Ideally in free steam at 100°C. Alternatively in a waterbath just less than boiling.
It is not recommended to heat bottled agar in a microwave oven or on a hot plate because there is a risk of serious injury from broken glass and hot agar.
We can provide a great deal of information so that you can make an informed choice.
Our Growth Media Types page provides information on the different types of prepared media and how they are used in environmental monitoring, sterility testing and process validation as a starting point.
For further information on other media and applications, please phone or email us with details.
The sterility of the packaged medium is assured and all but the outer layer of packaging is also sterile. Thus the risk to the environment to be sampled is greatly reduced. There is an additional benefit that the additional packaging and process extends the room temperature shelf life. This can be sufficient reason for small or irregular users to prefer irradiated.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
The storage condition for the majority of our prepared media is Ambient not exceeding 25ºC, the exception being a couple of very specialist products.
We have never specified storage in a fridge for our general media as this causes excessive condensation and can result in a very wet agar surface. This makes the product impossible to use.
View our data sheet on Redipor Storage Conditions.
General purpose media have nutrients that support the growth of most non fastidious culturable microorganisms. Selective growth media contain components that will inhibit the growth of some types of microorganisms, while supporting the growth of others.
General purpose media, such as Tryptone Soya Agar, are used to produce total counts. While selective media, such as XLD for Salmonella species, are used to test presence/absence of specific types of microorganism.
CHERWELL IS DIFFERENT
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust















