Incubation of plates is vital as part of environmental monitoring, product testing, batch control, or for media or disinfection qualification. An incubation failure potentially puts the validity of results at risk, as well as being time-consuming, costly and frustrating.
Microbial growth and morphology are impacted by both the time of incubation and the temperature to which they are exposed. These factors can depend on the media used, which will be dictated by the incubation regime elected.
Equipment validation is critical
An incubator should have sufficient space and ability to maintain temperature within the unit. Validation should prove this is achieved, using sufficient temperature mapping to cover all shelves and corners of the incubator. This validation process should identify any out-of-tolerance areas within the incubator, enabling operators to ensure these are not used.
Fan-assisted incubators should see relatively consistent distribution of heat, but this can be compromised by the number of products placed within the unit, or the location of the fan. The location of the fan and the heat source must be identified to reduce risk of agar desiccation.
Maintenance and re-qualification schedules should be in place to ensure equipment remains fit for purpose. Poor maintenance or change of incubator use can affect microbial growth if the change is not appropriately controlled. Using separate incubators for different operational functions, and removing the risk of cross-contamination of plates, is good practise. Plates from environmental monitoring programmes should be placed in separate incubators to other growth promotion studies, especially if high numbers of bacteria are expected.
Choosing the correct incubation regime
Dual incubation regimes must be validated. The variation in which time and temperature are used as the first and second phases may be dependent on the typical microflora within the area being monitored. Dual incubation consists of exposure at 30-35°C, and exposure at 20-25°, for a defined period, but collectively the exposure period would be seven days.
Management of dual incubation needs consideration, either by using dual incubators or removing plates from one incubator to another. The lag time between temperatures also needs consideration. The type of bacterial growth expected can be a factor as to which temperature is chosen first: as some viable organisms may become stressed and not grow when exposed to higher temperatures, resulting in an inaccurate count.
Over-colonisation or spreading of faster-growing or motile bacteria and/or fungi should also be taken into account to ensure the incubation temperature does not allow these to out-compete or suppress the growth of other microorganisms available on the plate.
Does Incubation time affect results?
Incubation time and the point at which plates are inspected can be critical. If plates are incubated for five days, these may need to be checked daily to enable accurate counts. Leaving plates for five days without inspection could impact the accuracy of counts if plates become overgrown with dominant bacteria.
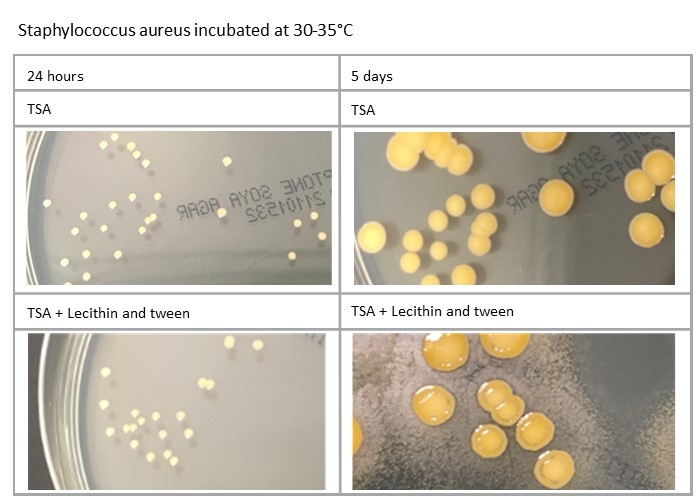
Plates’ incubation time can also impact on the morphology: for example, Staphylococcus aureus and Staphylococcus epidermidis will be different at five days compared to 24 hours. Variations in media formulation, such as TSA against TSA with lecithin and tween, can also have an effect, as demonstrated here:
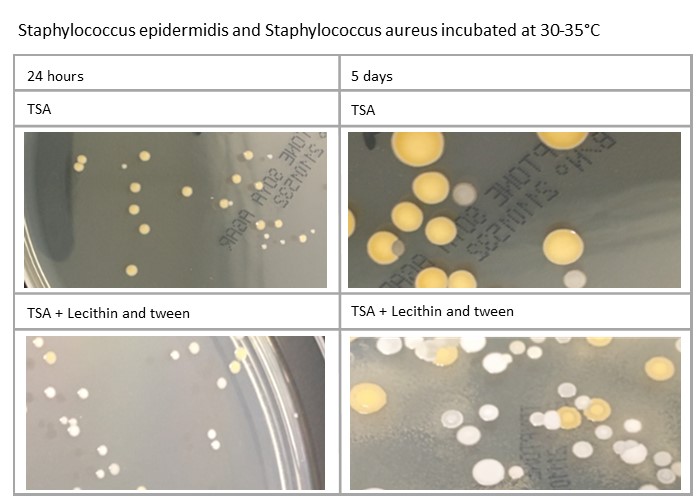
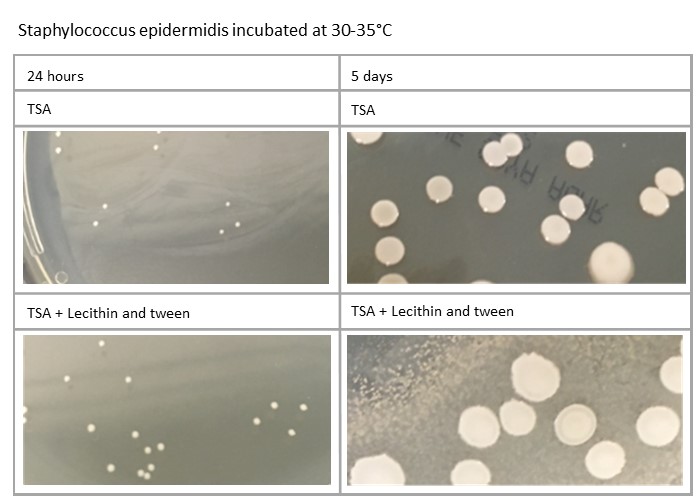
Variation in process can affect incubation of plates. How plates are managed in relation to incubation at weekends, when staff may not be present, should also be considered, as should public holidays, when incubation time may be further increased.
Importance of plate incubation should not be underestimated
The incubation of plates and the daily use of incubators may not always be a consideration when risk assessing process. Mitigation of risk is an easy process, achieved through planned maintenance and requalification. Issues can occur through operator error, however. Understanding the morphology in relation to incubator time and temperature can help when reviewing root cause and corrective actions associated with out-of-trend data.