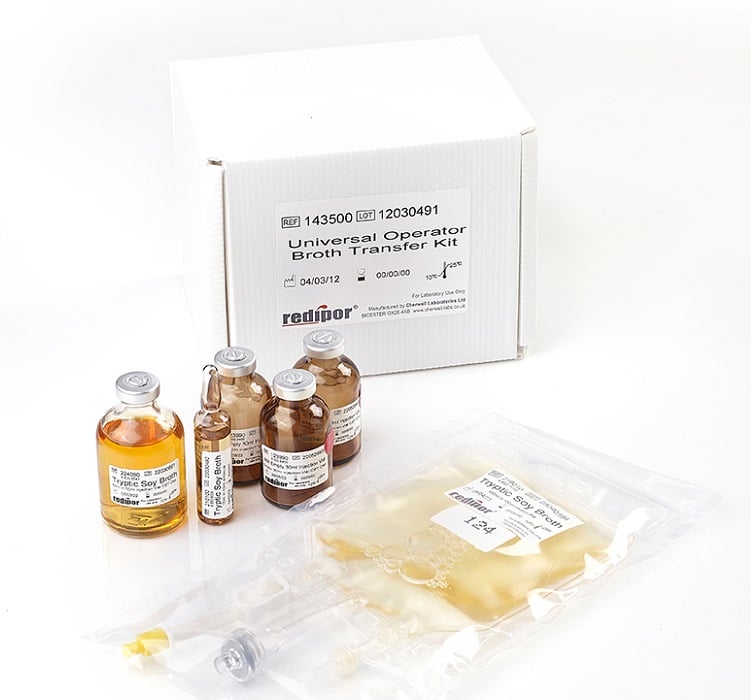
A kit containing vials, ampoule and broth bag to assess operator technique
Redipor® Universal Operator Broth Transfer Kit
The Redipor® Operator Broth Test Kit is a pre-packaged set of components; created in accordance with guidance from the UK Pharmaceutical Aseptic Services Committee; to aid in the assessment of operators undertaking aseptic preparations.
Used in high level cleanroom environments as a standard assessment of an operator's ability to maintain the sterility of materials during the preparation of aseptically prepared injectable dose forms, and for routine operator monitoring.
The kit contains: a 50ml Tryptic Soy Broth (TSB) injection vial; three empty 30ml sterile injection vials; a 100ml TSB broth bag and one 10ml TSB ampoule.
The EVA infusion bag is particularly appropriate to TPN compounding. All components are supplied by Cherwell Laboratories in a purposefully designed corrugated plastic box, for ease of use.
Redipor Universal Broth Transfer Kit Data Sheet

A TITLE THAT WILL DESCRIBE YOUR CONTENT OFFER
WSB - Content Section Ten
The game's not big enough unless it scares you a little. Now we know what they mean by 'advanced' tactical training. A surprise party? Mr. Worf, I hate surprise parties. I would *never* do that to you. Your head is not an artifact!
redipor universal operator broth transfer kit
Each compact, easy to store kit contains:
3 x Empty 30ml Sterile Injection vials
1 x 50ml Tryptic Soy Broth (TSB) injection vial
1 x 10ml TSB Ampoule
1 X 100ml TSB broth bag

Desiccation of Agar – Choosing Appropriate Media Fill Volume
In this animation Cherwell explains why desiccation and cracking can occur on plates, and why a deeper fill volume may help reduce the impact in your environmental monitoring environment.
What our customers say
Will Brearley, Administrator
Helvic Laboratories
"Alex in sales is fantastic, Hamish has been super helpful too and I've never had a bad experience. Big thanks to Shareen for completing an NC in about 3 hours today too! Saved us a huge headache down the line and we can't thank you all enough. Easily the best supplier we have - there's a reason we rely on you for our most critical media."
Quality Assurance and Cleanroom Specialist
Manufacturer and Supplier of Biocidal and Cleaning Products for Cleanrooms
"Customer service is excellent and I always receive a swift response from one of the team. Should I request a price - I am then provided with information regarding any costs and an estimated delivery date. Once an order is placed, it is always on time. Any queries are quickly resolved. It is a pleasure doing business with Cherwell - it really feels like you care about the customer."
Kieran Broadbridge, Senior Project Leader
Micronclean
"Hassle free. Quality product. Minimal QC issues with good communication when they do occur."
Quality Manager
BioDivide Ltd
"Great customer service and always keeping our company informed of changes particularly concerning regulatory matters that could impact us. Helpful, friendly, efficient."
need something else?
Browse our products and services
FREQUENTLY
Asked Questions
For Redipor media the Use By date is the last day on which the medium should be inoculated or exposed. Any incubation period that is normal for that product may begin on that day. For example a 14 day sterility test in TSB or a 5 day incubation for a TSA settle plate.
The majority of our prepared media can be stored in ambient conditions, not exceeding 25ºC. There are only a small number of very specialist products that require different storage conditions.
We have never specified refrigeration as a storage condition for our general media as this causes excess condensation and can result in very wet agar, rendering it impossible to use.
Our Redipor Prepared Media range is so extensive that not every product is included in the official price list. In addition to offering such a wide selection of products, we have developed a flexible manufacturing process enabling us to produce bespoke solutions.
Find out more information here.
Contact us to discuss your requirements, whatever they are.
View our data sheet on recycling Redipor products.
AnalytiChem UK is different
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust















