


gamma irradiated prepared media plates for environmental monitoring in pharmaceutical manufacture, cleanrooms and more
Gamma Irradiated Prepared Media
In addition to our standard range of Redipor® media products, we also offer triple-wrapped irradiated settle plates, 90 and 140mm Petri dishes and contact plates. Gamma irradiation extends shelf-life typically to 6 months in ambient conditions.
All irradiated products are subjected to the same rigorous quality checks as our standard pre-poured plates. Once packed, they then pass through an additional sterilisation process.
Finally, they are tested after irradiation to confirm growth properties and pH. A detailed certificate of analysis is provided with each batch.
GS1 data matrix code labels can be added to our plates at your request. The use of barcodes allows for automated scanning and data capture, within Laboratory Information Management Systems (LIMS), which enhances accuracy.
Packaging plays an important role in maintaining conditions for the agar and providing consistent, reliable prepared media that delivers confidence in your sampling.
The Redipor triple-wrapped irradiated plates packaging illustration demonstrates the sterility assurance provided by each layer, following exposure to gamma radiation. It shows how the packaging allows the plates to be easily handled, while being transported through each stage of a facility, without the risk of introducing contaminants.
Redipor Irradiated Media Data Sheet
Redipor Neutralisers Data Sheet
Redipor Barrier Pack Data Sheet
Redipor Plate Specifications Data Sheet
Safety Data Sheet - Redipor Plates
ANALYTICHEM UK GAMMA IRRADIATED MEDIA AND PACKAGING
Media and packaging solutions: contact us to place your order
Irradiated media – Barrier pack
For use in environments where Vaporised Hydrogen peroxide (VHP) is deployed, such as Isolators, Redipor Barrier pack provides a validated, protective layer to prevent the ingress of VHP gas. Available for both 90mm Petri dishes and Contact plates, this product is supplied in standard sleeves of 10 plates.
Irradiated Petri dishes – 90mm
Irradiated Petri dishes – 140mm
Irradiated Contact plates
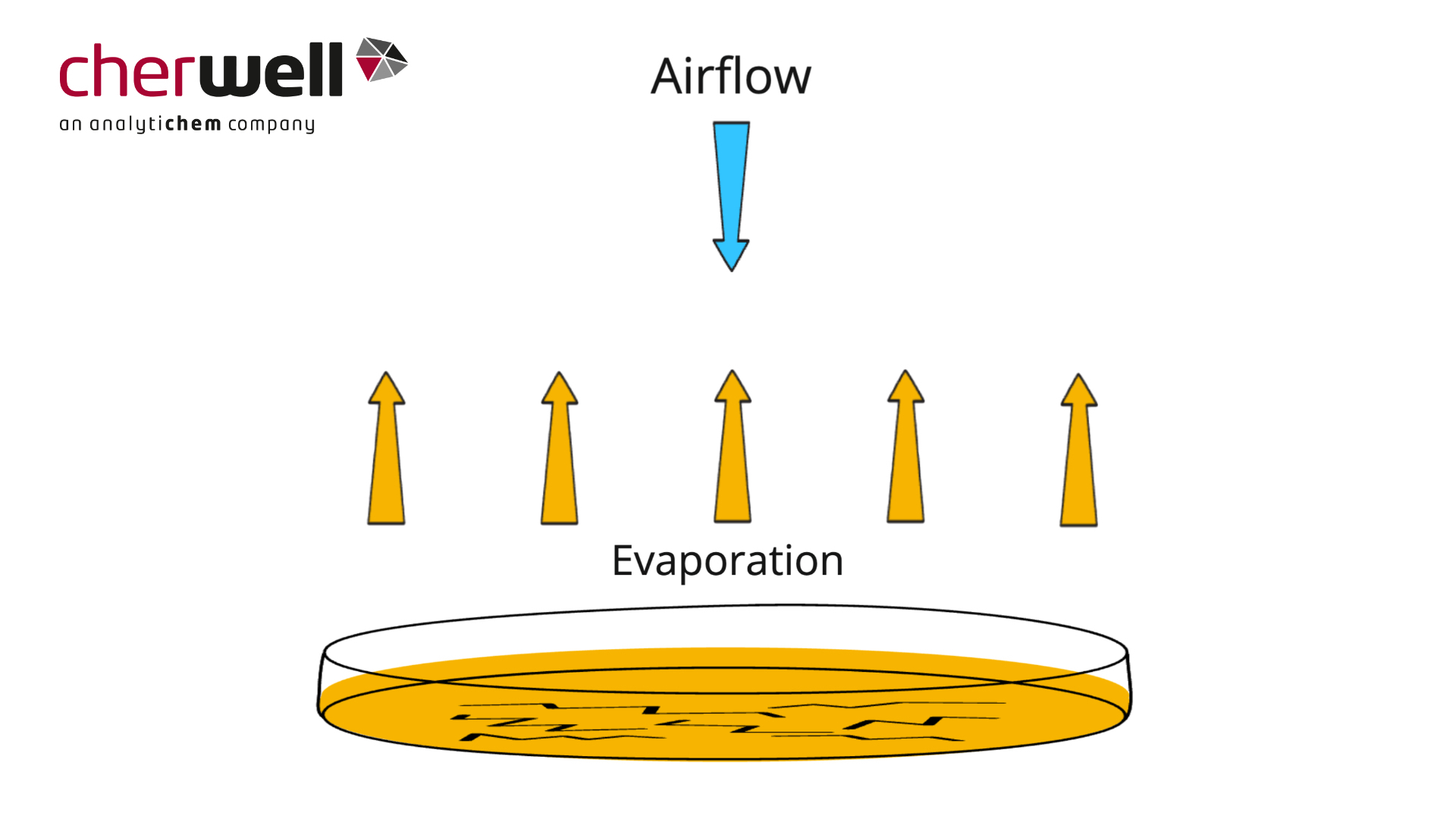
Desiccation of Agar – Choosing Appropriate Media Fill Volume
In this animation AnalytiChem UK explains why desiccation and cracking can occur on plates, and why a deeper fill volume may help reduce the impact in your environmental monitoring environment.
What our customers say
Tracey Roberts, Quality Assurance Technician
Wrexham Maelor Hospital
"Cherwell have always provided a great service, with innovative ideas and products. Great customer service too."
Gerald Prout
Kennet Bioservices Limited
"All my contacts with Cherwell have been resolved satisfactorily with 24-48 hours."
Microbiology Technician
Industrial Pharmaceutical Company
"The service from Cherwell is excellent, they will always try to provide help where possible."
Jane Maffei, Lead Technician in Aseptic Services
Worcestershire Royal Hospital
"Cherwell are reliable, adaptable, and nothing is to much trouble, excellent customer services. Friendly and helpful."
Head Microbiologist
North Tees and Hartlepool NHS Trust
"Thanks Emma – and all for your help. This does serve to back up the reason we have had such good relations with Cherwell for so many years. Proactive holistic bigger picture thinking."
Louisa Blake, Senior Pharmacy Technician QA/QC
Salisbury District Hospital
"You provide us with an amazing flexible service. Although you are a growing firm you still have the customer service of a dedicated provider! Thanks."
Facilities Coordinator
Roslin Cell Therapies Limited
"Cherwell provide a quick response, very good cooperation and availability, with precise service, high quality reports and overall performance."
Will Brearley, Administrator
Helvic Laboratories
"Alex in sales is fantastic, Hamish has been super helpful too and I've never had a bad experience. Big thanks to Shareen for completing an NC in about 3 hours today too! Saved us a huge headache down the line and we can't thank you all enough. Easily the best supplier we have - there's a reason we rely on you for our most critical media."
Sarah Stewart, Senior Microbiologist
Pharmaceutical CMO
"Super service - when we had an issue with our existing supplier of Contact Plates - you've stepped in and now we're going with you for this product."
Quality Assurance and Cleanroom Specialist
Manufacturer and Supplier of Biocidal and Cleaning Products for Cleanrooms
"Customer service is excellent and I always receive a swift response from one of the team. Should I request a price - I am then provided with information regarding any costs and an estimated delivery date. Once an order is placed, it is always on time. Any queries are quickly resolved. It is a pleasure doing business with Cherwell - it really feels like you care about the customer."
Kieran Broadbridge, Senior Project Leader
Micronclean
"Hassle free. Quality product. Minimal QC issues with good communication when they do occur."
Quality Manager
BioDivide Ltd
"Great customer service and always keeping our company informed of changes particularly concerning regulatory matters that could impact us. Helpful, friendly, efficient."
Technical and Regulatory Affairs Manager
Cleaning Products Supplier
"Thank you! Once again – great service! Goods received within 2 days of ordering and all packaging intact, CoAs received same day."
National Blood Service
Birmingham
"Your service has always been very good, as well as your documentation. Quite a lot of companies you have to chase these days for deliveries and certificates. You always send them promptly."
Julie Bowden, QA Releasing Officer
Pharmacy Manufacturing Unit, Portsmouth
"Friendly, helpful, efficient. Always get a prompt reply to any query. Always feel like a valued customer. Definitely feel like the company cares about it's customers and respects the industry we work in."
need something else?
Browse our products and services catalogue
FREQUENTLY
Asked Questions
The sterility of the packaged medium is assured and all but the outer layer of packaging is also sterile. Thus the risk to the environment to be sampled is greatly reduced.
There is an additional benefit that the additional packaging and process extends the room temperature shelf life. This can be sufficient reason for small or irregular users to prefer irradiated.
Our Redipor Prepared Media range is so extensive that not every product is included in the official price list. In addition to offering such a wide selection of products, we have developed a flexible manufacturing process enabling us to produce bespoke solutions.
Find out more information here.
Contact us to discuss your requirements, whatever they are.
The majority of our prepared media can be stored in ambient conditions, not exceeding 25ºC. There are only a small number of very specialist products that require different storage conditions.
We have never specified refrigeration as a storage condition for our general media as this causes excess condensation and can result in very wet agar, rendering it impossible to use.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
The final wrap of irradiated plates should be removed at the point of use and it is then a question of risk.
For example in a grade A isolator it might be reasonable to rewrap a part pack of plates for use in the next session. If all the exposed plates show no growth it is a reliable result.
However if one or more colonies appear on the plate it might not be possible to know during which session the contamination occurred and the OOT investigation becomes more complicated and expensive.
For Redipor media the Use By date is the last day on which the medium should be inoculated or exposed. Any incubation period that is normal for that product may begin on that day. For example a 14 day sterility test in TSB or a 5 day incubation for a TSA settle plate.
Not as obvious as it might sound. 3 layers of wrap between the agar plate and the outside world, of course, but Redipor can offer at least 3 different ways to achieve that with our standard product range.
We then have even more bespoke solutions to meet individual needs. Let us know how and where you want to remove the layers and we can find the best solution.
ANALYTICHEM UK IS DIFFERENT
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust

















