AIR SAMPLING SOLUTIONS FOR YOUR CLEANROOM
Reliable and durable active air monitors for pharmaceutical and healthcare settings

Explore our comprehensive range of SAS microbial air samplers, calibration services, and accessories tailored for the pharmaceutical, healthcare, and cleanroom manufacturing industries.
Designed for reliable environmental monitoring in controlled areas such as cleanrooms, operating theatres, isolators, and production zones, our solutions help ensure compliance and product safety.
Discover our full range of models and services.
ACTIVE AIR SAMPLING FOR FOOD SAFETY AND QUALITY
Protect your products and processes with trusted microbial monitoring solutions

Ensure the highest standards of hygiene and product integrity with our range of SAS microbial air samplers, accessories, and calibration services tailored for the food and beverage industry.
Our equipment supports effective environmental monitoring in production areas and other controlled zones—helping you meet regulatory requirements and protect consumer safety.
Explore our full range of models and services.

A TITLE THAT WILL DESCRIBE YOUR CONTENT OFFER
WSB - Content Section Ten
The game's not big enough unless it scares you a little. Now we know what they mean by 'advanced' tactical training. A surprise party? Mr. Worf, I hate surprise parties. I would *never* do that to you. Your head is not an artifact!
CHERWELL MICROBIAL AIR SAMPLERS
Air samplers for multiple application: contact us to place your order

Desiccation of Agar – Choosing Appropriate Media Fill Volume
In this animation Cherwell explains why desiccation and cracking can occur on plates, and why a deeper fill volume may help reduce the impact in your environmental monitoring environment.
What our customers say
Quality Manager - BioDivide
BioDivide Ltd
"Great customer service and always keeping our company informed of changes particularly concerning regulatory matters that could impact us. Helpful, friendly, efficient."
Julie Willis, Senior Assistant Technical Officer
NHS Blood and Transplant
"I am always pleased with the efficiency and quick turnaround time when I send our Air Samplers off to Cherwell for their service."
Thomas Mullen, Quality Operations Specialist (QA)
MedPharm Ltd
"I met with representatives from Cherwell at the Cleanroom Technology Conference recently, and found them to be not only approachable and friendly, but also highly knowledgeable and able to help with a query I had on microbial monitoring of compressed air."
Manager
Industrial Pharmaceutical Company
"Service from Cherwell is outstanding. The equipment is extremely reliable. That's what we ask for."
Julie Bowden, QA Releasing Officer
Pharmacy Manufacturing Unit, Portsmouth
"Friendly, helpful, efficient. Always get a prompt reply to any query. Always feel like a valued customer. Definitely feel like the company cares about it's customers and respects the industry we work in."
Microbiology Technician
Industrial Pharmaceutical Company
"The service from Cherwell is excellent, they will always try to provide help where possible."
NEED SOMETHING ELSE?
Browse our products and services section
FREQUENTLY
Asked Questions
Typical requirements suggest 1,000 litres per air sample in high risk areas, such as: grade A filling lines, grade B clean rooms, operating theatres etc. As the criticality of the area reduces, the sample size can be reduced. The aim is to achieve a representative sample; so where higher counts would be expected, a smaller sample produces a more realistic number of cfu to count.
SAS samplers were originally designed for Contact plates, however, a Petri dish option has been available for a number of years. It is really a personal choice, although this should be decided at time of purchase, as the sampler will be specifically configured for the plate type chosen. There are advantages for each version and we would be happy to discuss your specific needs.
No, do not put your SAS sampler in a steam autoclave. The only part that can be autoclaved is the drilled head. The unit can be wiped with alcohol wipes to decontaminate it. The only other exception is the SAS Pinocchio, parts of which can be autoclaved.
Analytichem UK recommends every 12 months and we will send a reminder for the month it is due. For some situations local procedures demand more frequent recalibrations so Cherwell is happy to offer tailored recalibration date labelling and reminders on request.
The sterility of the packaged medium is assured and all but the outer layer of packaging is also sterile. Thus the risk to the environment to be sampled is greatly reduced. There is an additional benefit that the additional packaging and process extends the room temperature shelf life. This can be sufficient reason for small or irregular users to prefer irradiated.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
The storage condition for the majority of our prepared media is Ambient not exceeding 25ºC, the exception being a couple of very specialist products.
We have never specified storage in a fridge for our general media as this causes excessive condensation and can result in a very wet agar surface. This makes the product impossible to use.
General purpose media have nutrients that support the growth of most non fastidious culturable microorganisms. Selective growth media contain components that will inhibit the growth of some types of microorganisms, while supporting the growth of others.
General purpose media, such as Tryptone Soya Agar, are used to produce total counts. While selective media, such as XLD for Salmonella species, are used to test presence/absence of specific types of microorganism.
ANALYTICHEM UK IS DIFFERENT
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust
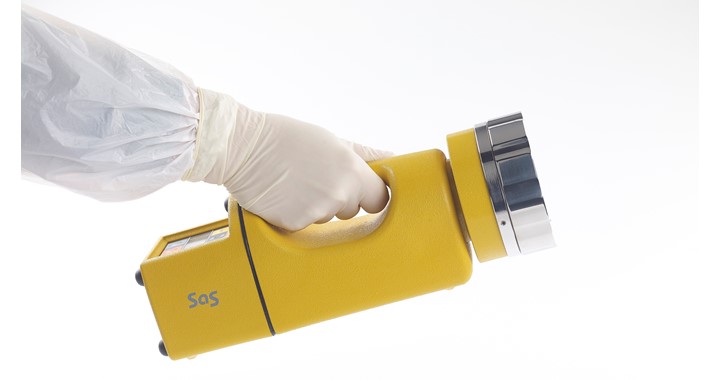
Air Samplers for Environmental Monitoring
Do you have the right air sampler for your needs?
Choosing the best air sampler means that compliance, efficiency and consistency are met with confidence.
Learn more about types of air samplers and how each works. Download technical datasheets for each model and brochures to help you make a selection.

















