Redipor® TwistLock
– Secure. Reliable. Efficient.
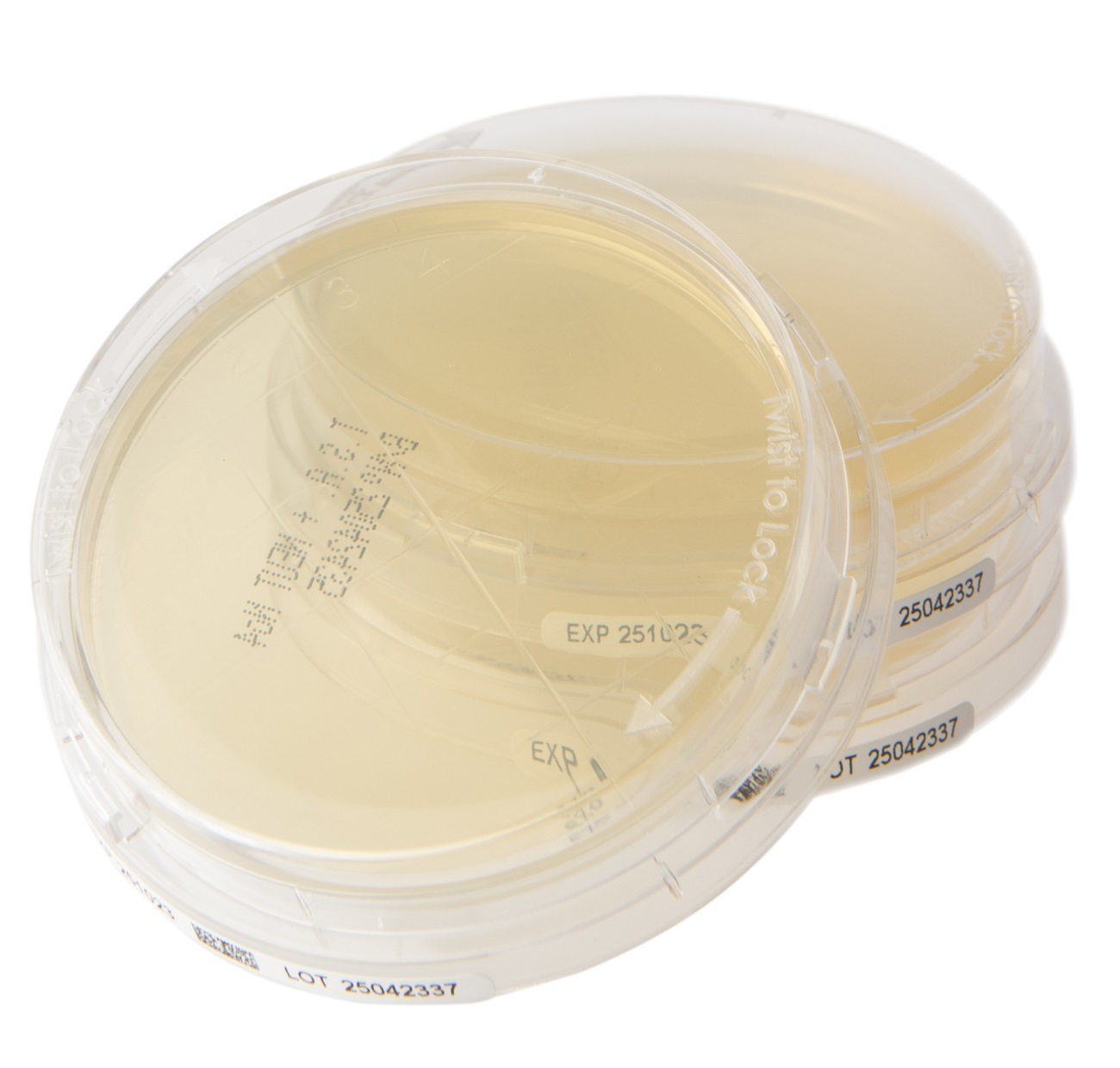
Redipor® TwistLock, a new range of plated prepared media designed to enhance sample security, data integrity, and workflow efficiency.
Introducing Redipor® TwistLock:
Analytichem UK's (formerly Cherwell) new range of plated prepared media designed to enhance sample security, data integrity, and workflow efficiency.
With a locking lid design and featuring a side-label with GS1 data matrix, these plates reduce contamination risks, improve traceability, and support automation—ensuring your environmental monitoring meets the highest standards.
In the pharmaceutical and medical manufacturing industries, the reliability of your environmental monitoring protocols is paramount.
One critical aspect of these protocols is the choice of the media used for sampling. Among the options available, plated prepared media with locking lids present a compelling alternative to standard plates.
KEY BENEFITS OF REDIPOR® TWISTLOCK
Why Choose Redipor® TwistLock?
Enhanced Contamination Protection
The secure locking lid prevents accidental opening, reducing exposure to airborne contaminants and ensuring more reliable microbiological testing.
Improved Sample Integrity
A tightly sealed environment prevents evaporation, sample loss, and cross-contamination, ensuring consistency throughout incubation.
Easy Handling & Transport
Designed for safe transport within and between facilities, the locking lid eliminates spillage risks, reducing the need for repeat sampling.
Long Shelf Life
Gamma irradiation ensures sterility, extending shelf life to six months—reducing ordering frequency, paperwork, and QC testing.
Reducing Condensation Issues
Our unique drying process prevents excess condensation after packing, keeping plates in optimal condition for use.
Isolator-Friendly Packaging
Available in Barrier Pack packaging, validated for VHP gassing cycles, ensuring safe decontamination for critical cleanroom environments.

DO LOCKING LID PLATES REALLY IMPROVE SAMPLE SECURITY?
The added security of Redipor TwistLock plates ensures
- More reliable microbial monitoring
- Reduces the risk of compromised data
- Minimises costly retesting
See how TwistLock locking plates compare to standard plates, in drop tests that simulate real lab accidents.
LEARN MORE ABOUT REDIPOR® TWISTLOCK
Discover more about the Redipor® TWISTLOCK
Optimized Workflow & Data Integrity
Redipor® TwistLock plates come with GS1 barcodes on side labels, allowing for:
- Fast, accurate scanning without repositioning plates
- Improved traceability and regulatory compliance
- Reduced manual errors and enhanced automation
- Seamless integration with LIMS and electronic batch records
- More precise colony detection as side labels keep the base clear, ensuring uninterrupted visual inspection or imaging if using an automated colony counter
Click the button below to watch our video that shows how our new Redipor® TwistLock plates can be scanned more quickly and efficiently compared with standard plates.

TAILORED TO YOUR NEEDS
The Redipor® TwistLock
- Neutralisers
Available with various neutralising agents to counteract disinfectants in your production environment. Need a custom formulation? Let’s talk. - Flexible Packaging Options
Choose from double wrap, triple wrap, or Barrier Pack, tailored for safe transfer into critical cleanrooms. - Deeper fill for high airflow environments
(e.g., isolators, safety cabinets) - 27ml fill Petri dishes for high airflow environments
(e.g., isolators, safety cabinets) - Customisable Plate Fill Volumes
Fit for Purpose. Fit for Your Purpose.
With Redipor® TwistLock, you get a secure, reliable, and efficient solution for pharmaceutical environmental monitoring. Upgrade your workflow today.
NEED SOMETHING ELSE?
Browse our products and services catalogue
FREQUENTLY
Asked Questions
SAS samplers were originally designed for Contact plates, however, a Petri dish option has been available for a number of years. It is really a personal choice, although this should be decided at time of purchase, as the sampler will be specifically configured for the plate type chosen. There are advantages for each version and we would be happy to discuss your specific needs.
The sterility of the packaged medium is assured and all but the outer layer of packaging is also sterile. Thus the risk to the environment to be sampled is greatly reduced. There is an additional benefit that the additional packaging and process extends the room temperature shelf life. This can be sufficient reason for small or irregular users to prefer irradiated.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
The storage condition for the majority of our prepared media is Ambient not exceeding 25ºC, the exception being a couple of very specialist products.
We have never specified storage in a fridge for our general media as this causes excessive condensation and can result in a very wet agar surface. This makes the product impossible to use.
General purpose media have nutrients that support the growth of most non fastidious culturable microorganisms. Selective growth media contain components that will inhibit the growth of some types of microorganisms, while supporting the growth of others.
General purpose media, such as Tryptone Soya Agar, are used to produce total counts. While selective media, such as XLD for Salmonella species, are used to test presence/absence of specific types of microorganism.
Our Redipor Prepared Media range is so extensive that not every product is included in the official price list. In addition to offering such a wide selection of products, we have developed a flexible manufacturing process enabling us to produce bespoke solutions.
Find out more information here.
Contact us to discuss your requirements, whatever they are.
Settle plates are used to monitor the level of viable particles in the environment through a process of passive air sampling. A viable particle settles on agar plates at a rate dependent on its characteristics and the airflow in the environment.
EU GMP Guide Annex 1 has recommended that 90mm settle plates can be exposed in cleanroom environments for up to 4 hours. However, agar plates may dry out during long exposures where the rate of air exchange is high. So, it might be necessary to use deep filled settle plates, or replace the settle plate after a shorter time to ensure satisfactory growth promotion after exposure.
For Redipor media the Use By date is the last day on which the medium should be inoculated or exposed. Any incubation period that is normal for that product may begin on that day. For example a 14 day sterility test in TSB or a 5 day incubation for a TSA settle plate.
ANALYTICHEM UK IS DIFFERENT
Supplying products is only the start
The best products prepared for you
Our Redipor® media products are batch tested, QC certified and delivered when you need them
Bespoke accessories and services
We provide additional equipment or services to make your environmental monitoring more effective
Best customer service
We build long-standing client relationships on understanding, commitment and trust



















